Stem cells and their remarkable power to transform into other types of cells have been the topic of numerous studies in recent years. One of the areas researchers have been looking at is whether these cells could help them identify therapies for the treatment of the various types of dementia.
Globally, around 50 million people suffer from dementia, according to the World Health Organization. Naturally, there is great interest in research that shows promise in curing the condition, but to date, there is no FDA approved therapy for Alzheimer’s or other types of dementia.
At this point, stem cells merely represent an area of research when it comes to treating dementia and other neurological disorders. Much of the funded research involves adult stem cells and induced pluripotent stem cells, while a select few involve embryonic stem cells.
What Does the Research Say?
Adult stem cells can come in different forms such as induced pluripotent stem cells (iPSCs), neural stem cells (NSCs) or mesenchymal stem cells (MSCs). While iPSCs are created in a lab using human tissue such as skin cells, NSCs come from the brain and MSCs are commonly found in bone marrow, adipose tissue or peripheral blood.
A recent analysis of stem cell studies in the journal Stem Cell Research & Therapy found that therapeutics derived from MSCs have performed the most consistently in clinical trials. Research around the impact of MSCs has now moved onto human clinical trials, with researchers optimistic about the possibility of eventually treating conditions such as dementia.
However, the analysis concluded that trials done using mice are “inadequate for predicting human clinical outcomes.” Therapies intended to treat Alzheimer’s will first have to prove successful in higher-order animals that more closely mirror human characteristics.
With that said, these kinds of neuro-replacement therapies have shown potential for the temporary enhancement of aging neuronal circuits in the brain. They may not be able to completely compensate for progressive neuronal loss, but they still have the potential to improve cognitive function and quality of life.
The Protein Effect
As researchers have learned more about the characteristics of Alzheimer’s patients’ brains, they have observed certain cellular behavior patterns unique to the condition.
One of those behaviors is in regard to the processing and elimination of two toxic proteins, amyloid beta and Tau protein, which clump together and cause the type of cell death seen in Alzheimer’s patients. This is due to a problem with the cell’s endosomal network, the system that moves proteins through a cell to process and recycle or dispose of it. When the system isn’t working properly, the proteins are not recycled or eliminated.
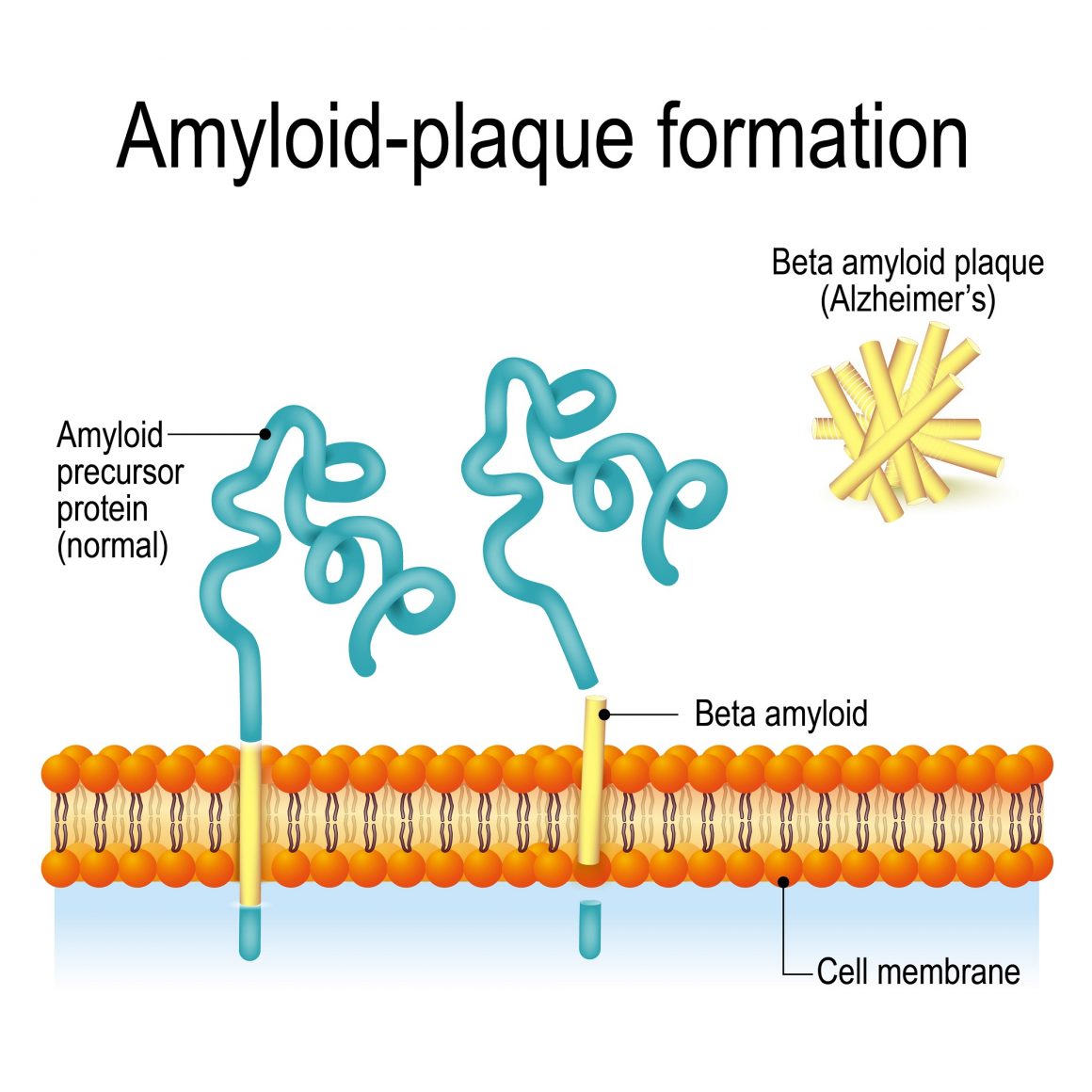
A study published last year by researchers at the University of Washington poses the idea that stem cells can be used to create new brain cells which lack this defect through the use of a compound that boosts endosomal function significantly.
To do this, the researchers took skin cells from patients diagnosed with Alzheimer’s or other types of dementia and reprogrammed them to act as stem cells, or in other words, they turn them into iPSCs. But given that these cells come from the person they would be delivered to, they would possess the same genetic mutation as the person’s current cells.
Scientists had to then experiment with compounds that support healthy endosomal function and found it in the form of compound called “R33.” The compound sufficiently supported the cell’s endosomal function, causing it to aggregate less of the Tau protein. They also tested the idea that the amyloid beta’s presence increases production of the Tau protein and found that the two did correlate.
They are currently working to on genetic modifications that could cause decreased production of the amyloid beta protein to begin with.
“The findings suggest that something upstream is affecting the production of amyloid beta and phosphorylated-Tau independently,” the study’s lead author Jessica Young said in a video interview with UW Medicine Seattle. “So one thing we’re going to work on going forward will be using these cell lines to identify what this upstream defect might be and whether it, too, could be a target for new therapeutics to treat Alzheimer’s.”